CLICK THE BACK BUTTON TO RETURN TO COURSE
To ensure proper use and monitoring of topical therapy side effects
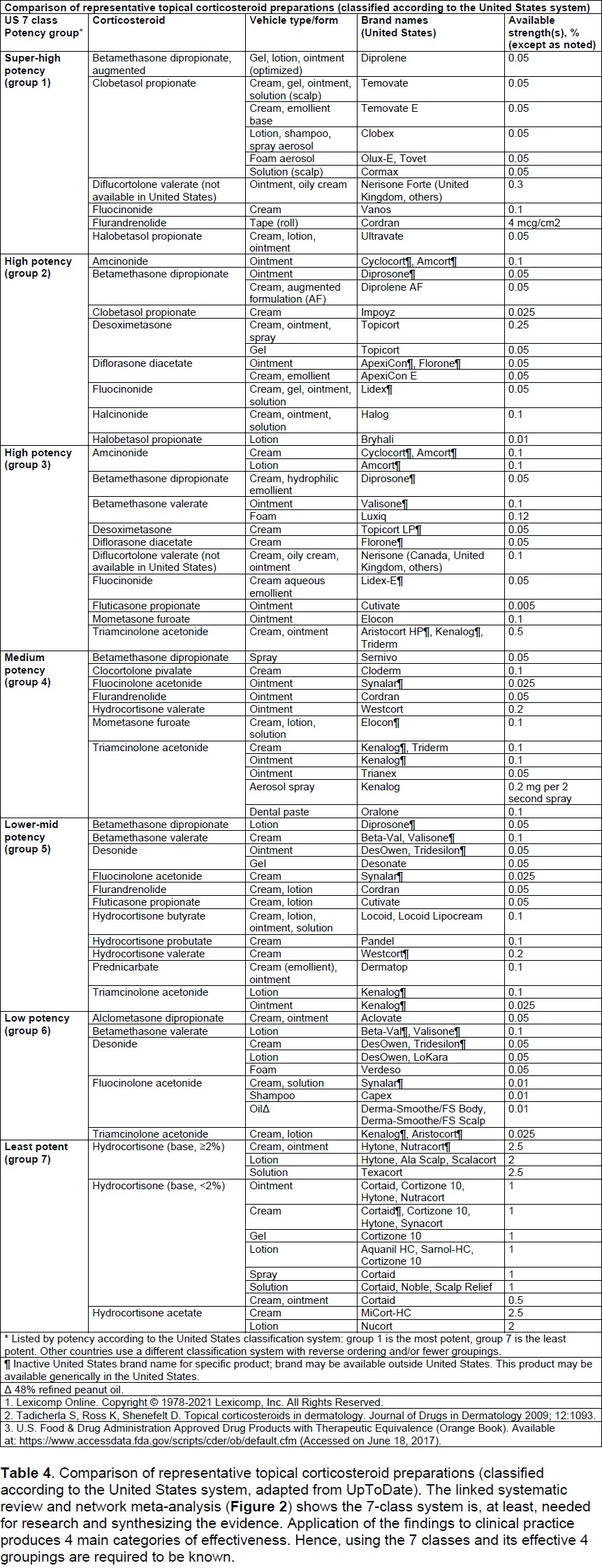
1. Know the potency of topical corticosteroid that your patient is using by downloading a potency chart and identifying the potency your patient is using.
2. Review Recommendations 3, 4 and 6 of the Atopic dermatitis: A practice parameter draft update 2023 or Summary section 14-18 of the Atopic Dermatitis Practice Parameter from 2012: ![]() Atopic dermatitis: A practice parameter DRAFT update 2023
Atopic dermatitis: A practice parameter DRAFT update 2023![]() Atopic dermatitis: A practice parameter update 2012
Atopic dermatitis: A practice parameter update 2012
Atopic Dermatitis: A practice paramter DRAFT update 2023
The guidance from the most recent practice parameters are currently in draft status with ongoing feedback followed by revision and are subject to change within this section of the CME as updates occur.
Recommendation 3: If AD is not controlled by moisturizers alone, then the clinician should recommend a topical corticosteroid. (Strong Recommendation, high certainty of evidence). (A)
Recommendation 4: In patients aged 3 months or older with uncontrolled atopic dermatitis refractory to moisturization alone, the JTF panel recommends the addition of a topical calcineurin inhibitor (Strong Recommendation, high certainty of evidence).
Recommendation 5: In patients with localized uncontrolled atopic dermatitis refractory to mid-high potency topical treatment the JTF panel suggests addition of a time and body area-limited (e.g. 4-7 days; minimum 1 hour to maximum overnight, once per day) trial of occlusive (wet wrap) low-mid potency topical corticosteroid therapy over continued standard topical therapy alone (conditional recommendation, very low certainty evidence).
Recommendation 6: In patients with uncontrolled atopic dermatitis using mid to high potency topical treatments (tacrolimus, topical corticosteroid US class 1-5) the JTF panel suggests applying the medication once per day over twice per day (conditional recommendation, moderate certainty of evidence).
Atopic Dermatitis: A practice parameter update 2012
Summary Statement 14: If AD is not controlled by moisturizers alone, then the clinician should recommend a topical corticosteroid.(A)
Summary Statement 15: Low-potency corticosteroids are recommended for maintenance therapy, whereas intermediate and high-potency corticosteroids should be used for the treatment of clinical exacerbation over short periods of time. (A)
Summary Statement 16: Clinicians should not prescribe potent fluorinated corticosteroids for use on the face, eyelids, genitalia, and intertriginous areas or in young infants. (D)
Summary Statement 17: Clinicians should recommend ultrahigh-potency corticosteroids only for short periods (1-2 weeks) and in non-facial non-skinfold areas. (D)
Summary Statement 18: When prescribing topical steroids, clinicians should remember that the degree of corticosteroid absorption through the skin and hence the potential for systemic adverse effects are directly dependent on the surface area of the skin involved, thickness of the skin, the use of occlusive dressing, and the potency of the corticosteroid preparation.(D)
Corticosteroids are effective medications for the treatment of AD. However, patients should be carefully instructed in their use to avoid potential adverse effects. Certain areas, including the mucous membranes (lips), genitalia, eyelids, face, and intertriginous areas, have increased potential for transepidermal corticosteroid penetration, and for this reason, potent fluorinated corticosteroids should be avoided in these areas. A low-potency corticosteroid preparation is recommended for these areas. For patients with very severe AD, clinicians might consider a few days of higher-potency topical steroids but should warn patients about local side effects and prescribe a limited amount of the topical steroid. Cheilitis can be problematic in patients with AD, and a few days of 1% or 2.5% hydrocortisone ointment (e.g, CortiBalm; Dr. Dan’s Lip Balms, Milan, Ind) followed by frequent use of moisturizers is usually effective.
Patients should be instructed to apply topical corticosteroids to skin lesions and to use moisturizers over uninvolved skin. There are 7 classes of topical corticosteroids ranked according to their potency based on vasoconstrictor assays. Some of the commonly used ones are listed in Table E1. Group I includes the super-potent or ultra-high potency topical corticosteroids with the greatest potential for adverse effects, both localized and systemic. Group VII includes the least potent topical corticosteroids and, as a group, has the least potential for adverse effects. More potent topical corticosteroids can be used for several days in non-facial non-skinfold areas to treat acute rashes. Patients should then be instructed to reduce the potency of topical corticosteroids applied to the skin. Because of their potential adverse effects, the ultra-high potency corticosteroids should be used for only short periods of time (1-2 weeks) and not on facial or skinfold areas. The high potency corticosteroids should only be used for short periods of time (up to 3 weeks) for clinical exacerbations. Intermediate potency corticosteroids, such as 0.1% triamcinolone, can be used for longer periods of time to treat chronic AD involving the trunk and extremities. Corticosteroids in gel formulations can contain a propylene glycol base that can irritate the skin, in addition to promoting dryness, limiting their use to the scalp and beard areas. In general, compared with topical creams, ointments have enhanced topical potency, although some modern vehicles might offset this tendency.
Adverse effects from topical corticosteroids are directly related to the potency ranking of the compound and the duration of use. It is incumbent on the clinician to balance the need for therapeutic potency with the potential for adverse effects. Adverse effects from topical corticosteroids can be divided into local and systemic adverse effects. Systemic adverse effects, which occur rarely, include suppression of the hypothalamic-pituitary-adrenal axis. Local adverse effects include the development of striae and atrophy of the skin, perioral dermatitis, rosacea, and allergic contact dermatitis (caused by the vehicle or steroid itself). Systemic adverse effects are related to the potency of the topical corticosteroid, the site of application, the occlusiveness of the preparation, the percentage of the body covered, and the length of use. The potential for prolonged use of potent topical corticosteroids to cause adrenal suppression is greatest in small children and infants.64,65 Two topical corticosteroids (fluticasone propionate and mometasone furoate) appear to have less systemic absorption and an efficacy profile that allows them to be used once as opposed to twice daily.66,67 Furthermore, several trials support that once control of AD is achieved with a daily regimen of topical corticosteroid, long-term control can be maintained with twice-weekly applications of topical fluticasone propionate to areas that have healed but are prone to eczema.68,69,70
It is recommended with any use of topical steroids to consider and discuss location of use, duration of use and potential side effects with acute or chronic use. Unlike high or ultra-high potency topical steroids, medium potency steroids have the benefit of maintenance therapy use. This has been studied in fluticasone propionate lotion/cream with once daily or twice weekly use in chronic flared areas of the body with low rates of adverse effects and successful prevention of relapse or flare.
In addition to the topical corticosteroids, many nonsteroidal options are available:
Topical PDE-4 Inhibitor
The only currently available product is Crisaborole 2%. It is indicated for mild to moderate atopic dermatitis disease and is approved down to 3 months of life. The improvement with crisaborole is small but statistically significant and provides young patients or those individuals with mild disease who have a fear of topical corticosteroids. The most common adverse effects of crisaborole were local site irritation including burning, stinging and/or pain which can limit its usefulness.
The 2023 Atopic Dermatitis Practice Parameter Draft discusses use of crisaborole in recommendation 7 which says: In patient with mild-moderate atopic dermatitis refractory to moisturization alone, the JFT panel suggests adding topical crisaborole 2% ointment over usual care alone.
Topical Calcineurin Inhibitors
Three calcineurin products are available including pimecrolimus 1% cream, tacrolimus 0.03% and 0.1% ointment. All of these have shown a benefit in decreasing flares of atopic dermatitis and decreasing itch. Pimecrolimus 1% and tacrolimus 0.03% have been approved down to 2 years of age with tacrolimus 0.1% approved for use for those over the age of 16 years. The topical calcineurins have been studied for use twice a day for flares or for maintenance of relapse/flare two to three times a week. Though there is limited comparative data between the topical calcineurin inhibitors and topical corticosteroids, very high and ultra-high potency topical steroids (betamethasone dipropionate and clobetasol) were more effective than pimecrolimus 1%. Lower potency steroids and pimecrolimus show similar efficacy. Comparing the calcineurin inhibitors to themselves, tacrolimus 0.1% has been studied against pimecrolimus 1% with more significant efficacy.
Topical calcineurin inhibitors do cause local site reactions that are more common with initiation of therapy, but this does resolve with subsequent exposures including burning and/or stinging. Though tacrolimus shows more effectiveness compared to pimecrolimus, its vehicle is an ointment which may not be preferred by some patients due to greasy after effect.
There is an FDA black box warning on topical calcineurin inhibitors for an elevated risk of cancers with metanalysis showing an increased risk for lymphoma cancers alone though the overall risk of lymphomas does remain low.
Topical Janus Kinase (JAK) inhibitor
Topical ruxolitinib 1.5% cream is approved for short-term, noncontinuous chronic therapy for mild to moderate AD in individuals over the age of 12 years with treatment not to exceed 20% of the body surface area (BSA) due to risk of systemic absorption and limitation of class specific black box warnings including serious infections, mortality, malignancy, major adverse cardiovascular events and thrombosis. Ruxolitinib significantly improves skin based on the Investigator Global Assessment (IGA). In a randomized head-to-head trial comparing triamcinolone 0.1% cream, similar EASI (Eczema Area and Severity Index) improvement was noted however improvement in itch was better with ruxolitinib.1 There is not long-term data published yet but short courses of ruxolitinib can be beneficial as another nonsteroidal option in mild to moderate adolescent and adult patients with atopic dermatitis. Most common adverse effects are local irritation including stinging and/or burning that improves with subsequent use.
The JAK inhibitors both systemic and topical do carry a black box warning and monitoring and monitoring is recommended and limitation or prevention of systemic absorption for topical therapy is recommended. Prescriptions should be limited to 60g per week of use.
1. Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863-872.
3. Review Consensus-based European guidelines for treatment ofatopic eczema (atopic dermatitis) in adults and children: part I JEADV 2018; 32(5): 657-682.
4. Review AAD 2023 update on topical therapies in AD. Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023
5. Review Atopic Dermatitis Yardstick and Yardstick update from 2023![]() Atopic dermatitis yardstick
Atopic dermatitis yardstick
Atopic dermatitis yardstick update 2023
CLICK THE BACK BUTTON TO RETURN TO COURSE

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward