To improve the treatment of atopic dermatitis by understanding for patients with severe disease the systemic nature of atopic dermatitis including the immune dysregulation secondary to T cell activation to specific Th subsets that do vary based on ethnicity and include Th2 and Th22 phenotypes.
Review Atopic Dermatitis Yardstick Update
Oral Immunosuppressants1: While many of the immunosuppressants are still being utilized or in some instances required for a trial prior to initiation or coverage of biologic/small molecule options, many of these products do have significant risks associated and although they do show improvement in atopic dermatitis, these medications should be used with caution and after thorough discussions with the patient. These medications are included here in case they are required to be utilized and familiarize the clinician with the available options; however many of these medications would be used off label per FDA indications.
Atopic Dermatitis Practice Parameter Draft Update in 2023 Summary Recommendation 25: In patients with atopic dermatitis, the JTF panel suggests against using systemic corticosteroids.
Corticosteroids: For many years, oral corticosteroids were the only FDA approved systemic therapy for AD. The use of systemic corticosteroids, such as oral prednisone, might have previously been required in the treatment of severe chronic AD, although there is a paucity of controlled studies, despite previous widespread use of this therapy. The evolving literature on atopic dermatitis with novel therapies that have improved treatment outcomes with less systemic side effects has meant that oral corticosteroids use for atopic dermatitis should be limited as their benefit does not exceed their associated risk. In a double-blind, placebo-controlled, crossover trial of 26 children with severe AD, those receiving 4 weeks’ treatment with combined oral plus nasal beclomethasone dipropionate improved significantly more than those receiving placebo. No adverse effects were observed, but 24-hour urinary cortisol excretion was slightly reduced.
The PRACTALL consensus report states that in cases of acute flare-up, while patients might benefit from a short course of systemic therapy with corticosteroids, long-term use and use in children should be avoided.
A comparison study of oral prednisolone versus cyclosporine in adults found a high rebound exacerbation rate in patients treated with prednisolone in spite of the use of moderate potency topical steroids and emollients. Clinical improvement with systemic corticosteroids is often associated with rebound flaring of AD after discontinuation. If a short course of oral corticosteroid therapy is given for a patient with severe AD, it is important to taper the dosage as it is discontinued and intensified skin care with topical anti-inflammatory therapy should be instituted during the corticosteroid taper to suppress rebound flaring of AD.
Cyclosporine or Ciclosporin (Off Label Use): Cyclosporine is a systemic immunosuppressant that targets cytokine transcription and production in antigen presentation cells and T cells decreasing T cell activation and is the most common systemic medication used for atopic dermatitis outside of systemic corticosteroids. It is approved for use of AD in many European countries. It has a rapid onset of action compared to other non-steroidal therapeutic options however ongoing monitoring is required due to nephrotoxicity (electrolyte derangement affecting potassium and magnesium, hypertension, acute kidney injury), lymphoproliferative malignancies, and neurotoxicity. Use is recommended to be limited to 1 year due to the malignancy concern as well as close monitoring of labs and blood pressure after initiation.
Methotrexate (Off Label Use): Methotrexate is a folic acid antagonist that at low doses has been used off label in patients with refractory AD due to its immunosuppression properties with similar efficacy of azathioprine seen in randomized control trials though it does appear to be less effective than cyclosporine or dupilumab.2,3 Compared to corticosteroids or cyclosporine, the time to onset is much slower with average maximum effect not seen until 10 weeks after initiation. The most common side effects are gastrointestinal, headache and dizziness with less common adverse effects being hepatotoxicity and myelosuppression. Given that it is a folic acid inhibitor, individuals on methotrexate need to be supplemented with folic acid 1 mg on days that they do not receive methotrexate.
Atopic Dermatitis Practice Parameter Draft Recommendation 22: Suggests against the use of methotrexate.
Azathioprine (Off Label Use): Azathioprine inhibits purine synthesis as a 6 mercaptopurine analog that inhibits the lymphocyte cell cycle causing immunosuppression. Evidence for azathioprine is limited to small studies but seems to have similar effectiveness to methotrexate with mild to moderate improvement. The most concerning side effect that requires monitoring is the potential for myelosuppression, however gastrointestinal symptoms are the most common limiting effect. Given the metabolism of azathioprine in the liver by an enzyme, thiopurine methyltransferase (TPMT), which has loss of function mutations commonly in the general population, screening for TPMT enzyme function is recommended prior to initiation. Dosing is based on the TPMT level. Time of onset for azathioprine is delayed to 4-8 weeks after initiation. Azathioprine has been shown to be safe for long-term use in pediatric and adult patients.
Atopic Dermatitis Practice Parameter Draft update Recommendation 20: The panel suggests against using azathioprine
Mycophenolate mofetil (Off Label Use): Mycophenolate is an inhibitor of purine synthesis and thus lymphocyte proliferation through binding to inosine monophosphate dehydrogenase. Mycophenolate has been shown to be mildly effective in pediatric and adult populations with safe long-term use with minimal side effects. The most common adverse effect is gastrointestinal distress though there is data at higher doses used for transplant patients of increased risk for infections, myelosuppression and risk of neoplasia.
Biologic Therapies: Biologics and other small molecule agents have been instrumental in the treatment of moderate to severe atopic dermatitis making options available to treat patients whom are refractory to topical therapy but limiting the systemic side effects as outlined above with systemic immunosuppressants.
Dupilumab: Dupilumab was the first biologic therapy approved for AD and dramatically improved the systemic treatment options for patients suffering with AD. Dupilumab is a fully human monoclonal IgG4 κ antibody against the IL-4 receptor α thus inhibiting IL4 and IL13 signaling due to the IL4Rα used in both Th2 signaling cytokines. Dupilumab has the benefit of covering many comorbidities that occur alongside AD including severe persistent asthma, steroid dependent asthma, prurigo nodularis, eosinophilic esophagitis and chronic rhinosinusitis with nasal polyposis. Dupilumab use was shown in phase III randomized control trials (SOLO1 and SOLO2) as monotherapy and use with topical corticosteroids improvement in EASI scores of 51.3 and 65% respectfully at 52 weeks with similar efficacy seen in children aged 12 to 18 with onset of efficacy seen at 4 weeks of therapy.4,5,6 Dupilumab is approved down to 6 months of age and is administered as a subcutaneous injection with weight-based and age-based dosing. Most common side effect is site irritation however the most concerning and bothersome symptom being noninfectious conjunctivitis that is specific to dupilumab use in AD7,8. This conjunctivitis can be transient and can respond to topical ocular anti-inflammatory agents managed alongside ophthalmology colleagues without discontinuation though sometimes this is required. Long-term safety data is continually becoming available with continued benefit while on therapy including slight increased efficacy with long term use without any other notable side effects.7,8,9 Dosing does continue to show every 2 weeks is the most effective dose.
Tralokinumab: Tralokinumab was approved in 2021 for use in adults with AD. Tralokinumab is a humanized IgG4κ monoclonal antibody that targets IL13 and prevents binding to the IL13 receptor α1 and α2 resulting in complete IL13 blockade. In phase III trials (ECZTRA1 and 2) adults with moderate to severe AD treated with subcutaneous tralokinumab 300mg dosed every 2 weeks as monotherapy showed improvement in IGA and EASI75 at 16 weeks as well as safety to 52 weeks.10 Tralokinumab dosing can change to every 4 weeks if improvement has been noted at the 16-week mark. In phase 3 trials, tralokinumab maintained remission of atopic dermatitis after discontinuation for up to 36 weeks. Most common side effects with tralokinumab are local site reactions and similar or slightly reduced rates of conjunctivitis as seen previously with dupilumab.11 Long term studies have shown continued efficacy and safety with tralokinumab but are still being monitored.12
Oral JAK inhibitors: There is a class specific black box warning for all JAK inhibitors due to increased risk of major acute cardiovascular event (MACE), veno-thromboembolism (VTE) and malignancy. This was based on data from tofacitinib in rheumatoid arthritis.13 however there have been associated VTE and MACE in the oral JAK inhibitors used for AD. Prior to initiation of the oral JAK inhibitors, it is recommended to obtain baseline status for tuberculosis, hepatitis B/C as well as a baseline CBC with differential looking for absolute lymphocyte/neutrophil count. It is recommended to repeat a complete blood count, complete metabolic panel 4 weeks after initiation as well as a lipid panel 12 weeks after initiating therapy.
Abrocitinib: Abrocitinib is an oral JAK1 inhibitor that is approved for adolescents and adults with atopic dermatitis. It is approved as 100mg and 200mg doses once a day. It is recommended to start at the 100mg dose and escalate to the 200mg dose if failing therapy after 12 weeks. The oral JAK inhibitors have a very quick onset of improvement and notable improvement with EASI75 met in phase III trials (Jade MONO-1 and MONO-2) by 12 weeks and itch improved within 48 hours after initiation.14,15 Improvement was faster than dupilumab during clinical trials though dupilumab did reach similar statistical improvement in AD as the 100mg dose. Most common side effects are nausea, headache and nasopharyngitis. Cases of HSV were noted in the clinical trials and a not clinically significant transient thrombocytopenia at the 200mg dose resolved without therapy discontinuation. No episodes of MACE or VTE were reported in the clinical trial for abrocitinib or in the extension however patients with significant risk factors were excluded from participation. Due to the black box warning, it is important to discuss with patients who have risk factors for cardiovascular or thromboembolism regarding the benefits of JAK usage and the risk of oral JAK inhibitors. It is recommended that if this drug is initiated, the practitioner should utilize the minimum dose required.
Upadacitinib: Upadacitinib is an oral JAK1 selective inhibitor approved for the treatment of AD in adults and adolescents older than 12 years of age. Updacitinib has shown significant improvement in EASI75 and EASI90 or the percentage of people with 75% and 90% of their EASI scoring being 0 to 1 as well as improvement in itch with continued response to 56 weeks.16,17,18 The response to upadacitinib in regard to itch and improvement in skin is very rapid. It is approved as 15mg and 30mg dosing with a dose response however it is recommended to start with the 15mg dose and titrating upwards if lack of response by 12 weeks.16 A systemic review and metanalysis found that upadacitinib 30mg had the highest clinical response of all biologics and JAK inhibitors. Upadacitinib 15 mg was associated with higher clinical responsiveness than dupilumab 300mg.18 The most common adverse effect was upper respiratory tract infections, headache, nausea, acneiform eruptions. The major adverse effects of the oral JAK inhibitors are a class wide adverse effect including major acute cardiovascular event (MACE), venous thromboembolism (VTE), malignancy and increased infections. The more common adverse effects are increased upper respiratory tract infections and acneiform rashes. There were 2 reported cardiovascular and VTE in the phase III and extension trial for upadacitinib.
Atopic Dermatitis is a complicated systemic disease and the treatment approach needs to be multifaceted for appropriate patient-centered care. The therapies for atopic dermatitis have dramatically grown and the future remains promising for novel therapies. Clinicians play a significant role in understanding the basics of AD and offering many options to affected patients.
Recommendations Infographic
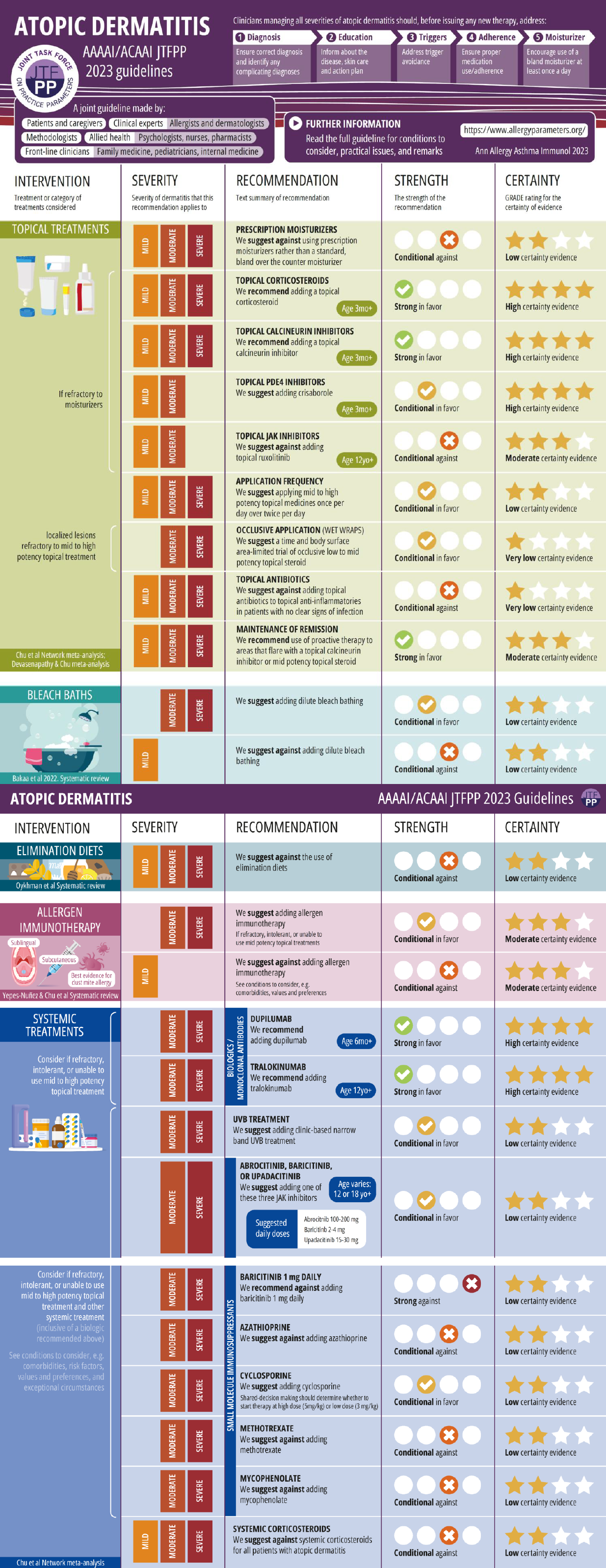
References
1. Drucker AM, Ellis AG, Bohdanowicz M, et al. Systemic Immunomodulatory Treatments for Patients With Atopic Dermatitis: A Systematic Review and Network Meta-analysis. JAMA Dermatol. 2020;156(6):659-667.
2. El-Khalawany MA, Hassan H, Shaaban D, Ghonaim N, Eassa B. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr. 2013;172(3):351-356.
3. Anderson K, Putterman E, Rogers RS, Patel D, Treat JR, Castelo-Soccio L. Treatment of severe pediatric atopic dermatitis with methotrexate: A retrospective review. Pediatr Dermatol. 2019;36(3):298-302.
4. Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and Safety of Dupilumab in Adolescents With Uncontrolled Moderate to Severe Atopic Dermatitis: A Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2020;156(1):44-56.
5. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2016;375(24):2335-2348.
6. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287-2303.
7. Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82(2):377-388.
8. Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-Term Efficacy and Safety of Dupilumab in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results Through Week 52 from a Phase III Open-Label Extension Trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23(3):365-383.
9. Beck LA, Deleuran M, Bissonnette R, et al. Dupilumab Provides Acceptable Safety and Sustained Efficacy for up to 4 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. Am J Clin Dermatol. 2022;23(3):393-408.
10. Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437-449.
11. Wollenberg A, Beck LA, de Bruin Weller M, et al. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. 2022;186(3):453-465.
12. Blauvelt A, Langley RG, Lacour JP, et al. Long-term 2-year safety and efficacy of tralokinumab in adults with moderate-to-severe atopic dermatitis: Interim analysis of the ECZTEND open-label extension trial. J Am Acad Dermatol. 2022;87(4):815-824.
13. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6(3):e001395.
14. Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87(2):351-358.
15. Meher BR, Mohanty RR, Padhy BM. Efficacy and safety of abrocitinib for the treatment of moderate-to-severe atopic dermatitis: a meta-analysis of randomized clinical trials. J Dermatolog Treat. 2022;33(4):2335-2343.
16. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials [published correction appears in Lancet. 2021 Jun 5;397(10290):2150]. Lancet. 2021;397(10290):2151-2168.
17. Silverberg JI, de Bruin-Weller M, Bieber T, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week 52 AD Up study results. J Allergy Clin Immunol. 2022;149(3):977-987.e14.
18. Silverberg JI, Hong HC, Thyssen JP, et al. Comparative Efficacy of Targeted Systemic Therapies for Moderate to Severe Atopic Dermatitis without Topical Corticosteroids: Systematic Review and Network Meta-analysis. Dermatol Ther (Heidelb). 2022;12(5):1181-1196

 Facebook
Facebook Twitter
Twitter LinkedIn
LinkedIn Forward
Forward